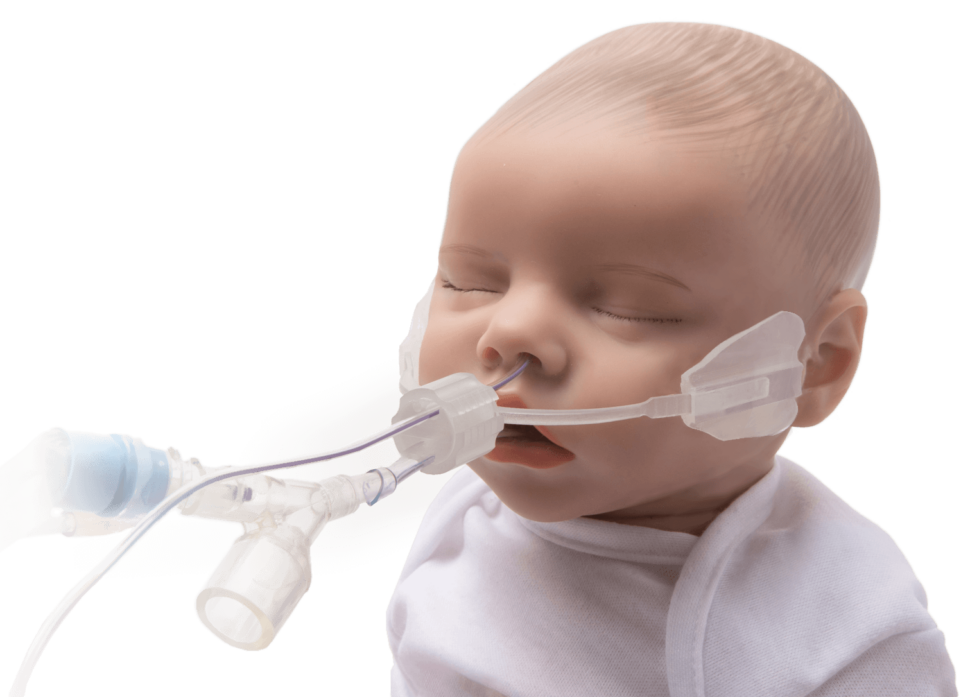
WarriorNP (an ADAPT Technology customer) ArcAngel’s Neonatal & Pediatric Endotracheal Tube
Developing and getting a medical product to market is an extraordinary journey filled with innovation, meticulous planning, and adherence to the highest standards. This process ensures that the product is safe, effective, and meets the needs of healthcare providers and patients. Let’s walk through the critical stages of medical product development, from the initial concept to market launch and post-market surveillance, with insights on how ADAPT Technology can help at every step.
1. Conceptualization and Feasibility
Conceptualization: It all starts with a spark of inspiration—identifying a medical need or a gap in the market. Understanding the clinical requirements and potential patient impact is crucial. Brainstorming sessions and consultations with medical professionals help in shaping a visionary product concept that addresses these needs.
Feasibility Study: Next, a feasibility study assesses whether this innovative concept is viable. This includes technical, clinical, and commercial evaluations to determine if the product idea is practical, can be developed with available technology, and has a promising market potential.
2. Preliminary Design
Preliminary Design: In this phase, detailed drawings and specifications for the product are created. This includes selecting materials, defining product features, and considering usability aspects. Engineers and designers work together to ensure the product is both functional and user-friendly. ADAPT Technology’s expertise in preliminary design ensures that every aspect of the product meets high quality and functionality standards.
3. Engineering Design
Engineering Design: Now, it’s time for detailed and precise designs. This phase involves creating technical drawings, schematics, and selecting appropriate materials and components. The goal is to translate the preliminary design into a detailed blueprint that can be used for prototyping. ADAPT Technology’s engineering design expertise ensures that designs are optimized for performance and manufacturability.
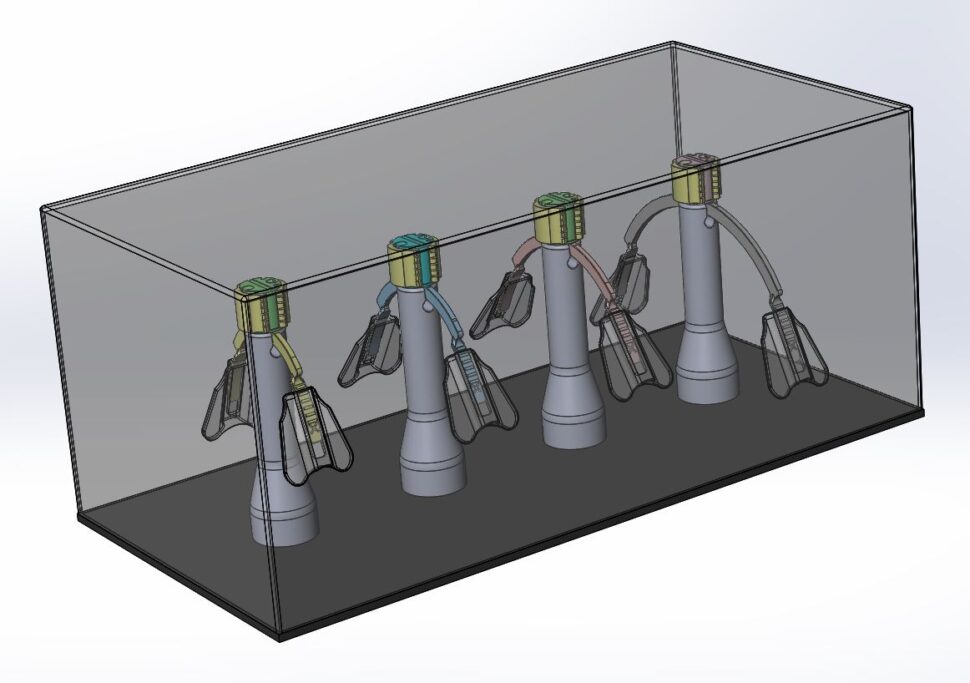
WarriorNP ArcAngel Neonatal and Pediatric Endotracheal Tube Holder
4. Prototyping
Prototyping: Prototyping is a crucial step where physical models of the product are created. With technologies like 3D printing, developers can quickly produce prototypes to test form, fit, and function. Multiple iterations are expected, with each prototype undergoing evaluation and refinement. ADAPT Technology’s advanced prototyping facilities and rapid iteration capabilities enable quick and effective testing and improvement of designs.
5. Engineering Development
Engineering Development: The engineering development phase begins after achieving a satisfactory prototype. This involves detailed engineering design, creating technical documentation, and preparing for production. Computer-aided design (CAD) software and simulation tools are often used to optimize product design. ADAPT Technology ensures rigorous testing and validation to meet all required specifications and standards.

6. Prototyping (Again)
Second Prototyping: A second round of prototyping is often necessary to refine the product based on feedback from the prototypes and the engineering development phase. This round ensures that all design elements are perfected and the product meets all functional and regulatory requirements. ADAPT Technology provides ongoing support during this phase, utilizing advanced prototyping technologies to refine and perfect the product.
7. Regulatory Approval
Preclinical Testing: Before a medical product can be tested in humans, it undergoes preclinical testing. This includes laboratory tests and animal studies to evaluate safety, efficacy, and biocompatibility. The data collected is critical for regulatory submissions.
Clinical Trials: Clinical trials test the product on human subjects in multiple phases. These trials gather data on safety, efficacy, and optimal usage conditions. Regulatory agencies, like the FDA, require comprehensive clinical trial data before approving a product for the market.
Regulatory Submission: The regulatory submission involves compiling all necessary documentation, including preclinical and clinical data, technical specifications, and manufacturing details, and submitting it to regulatory bodies for review. The approval process can be lengthy and require rigorous evaluation. ADAPT Technology’s regulatory expertise ensures all submissions are thorough and meet all regulatory requirements, facilitating a smoother approval process.
8. Manufacturing and Quality Control
Manufacturing Setup: Once regulatory approval is obtained, the focus shifts to setting up the manufacturing process. This includes selecting manufacturing partners, setting up production lines, and ensuring compliance with Good Manufacturing Practices (GMP). ADAPT Technology assists in scaling the manufacturing process, ensuring production is efficient and cost-effective while maintaining high-quality standards.
Quality Control: Maintaining high-quality standards is crucial in medical product manufacturing. Quality control measures include routine inspections, testing raw materials and finished products, and adherence to standard operating procedures (SOPs). A robust quality management system (QMS) helps maintain consistency and reliability. ADAPT Technology implements stringent quality control protocols to ensure every product meets the highest safety and efficacy standards.

BaxMax Back Support
9. Market Launch
Marketing Strategy: A successful market launch requires a well-planned marketing strategy. This includes identifying target markets, defining pricing strategies, and developing marketing materials. Engaging with healthcare providers, attending medical conferences, and leveraging digital marketing are effective ways to promote the product. ADAPT Technology’s marketing support services help clients develop and execute effective go-to-market strategies that maximize product visibility and adoption.
Distribution and Sales: Establishing a distribution network is essential for getting the product to market. This involves partnerships with distributors, setting up logistics, and training sales teams. Ensuring the product is available where it is needed most is critical to market penetration. ADAPT Technology’s extensive network and logistical expertise ensure that products reach their target markets efficiently and effectively.
10. Post-Market Surveillance
Monitoring and Feedback: Once the product is on the market, ongoing monitoring is necessary to track its performance. This includes gathering user feedback, monitoring for adverse events, and ensuring compliance with regulatory standards. ADAPT Technology provides comprehensive post-market surveillance services to ensure that products continue to perform as expected and that any issues are promptly addressed.
Product Improvements: Continuous improvements can be made to the product based on feedback and performance data. This may involve minor adjustments or significant upgrades to enhance functionality and user experience. ADAPT Technology supports ongoing product improvement efforts, leveraging its expertise to implement practical changes that enhance product performance.
Regulatory Compliance: Maintaining regulatory compliance is an ongoing process. This involves staying updated with regulation changes, conducting regular audits, and submitting necessary documentation to regulatory bodies. ADAPT Technology’s regulatory team ensures clients remain compliant with all applicable laws, providing peace of mind and allowing them to focus on their core business.

Best Practices and Challenges
Bringing a medical product to market involves navigating various challenges and adhering to best practices. One significant challenge is managing the cost and time associated with extensive testing and regulatory approval. Companies must balance innovation with compliance, ensuring groundbreaking and safe products.
Effective project management is crucial. Clear communication, defined milestones, and rigorous documentation help keep the development process on track. Collaboration between cross-functional teams, including engineers, medical professionals, regulatory experts, and marketers, enhances the development process and ensures that all aspects of the product are optimized. ADAPT Technology’s project management expertise ensures projects are completed on time and within budget, minimizing risks and maximizing success.
Additionally, leveraging data analytics can provide insights into market trends, customer needs, and potential areas for innovation. Data-driven decision-making helps tailor the product to meet market demands and stay ahead of the competition. ADAPT Technology utilizes advanced analytics to inform product development strategies and ensure products meet market needs.

Conclusion
Developing and bringing a medical product to market is intricate and demands a meticulous approach at every stage. From initial concept to post-market surveillance, each step is crucial in ensuring the product is safe, effective, and meets the medical community’s needs. Companies like ADAPT Technology play a pivotal role in this journey by providing comprehensive engineering solutions, regulatory expertise, and innovative approaches to product development. By adhering to best practices and leveraging technological advancements, we can bring groundbreaking medical products to market, ultimately enhancing patient care and healthcare outcomes.
If you are ready to bring your idea to life or want to revise a current product, you can contact us on our website or here.